Continuous Traits Are Often Influenced by Multiple Simple Mendelian Genes
Jeanne Kelly/National Cancer Institute.
When studying the relationships between genotype and phenotype, it is important to examine the statistical occurrence of phenotypes in a group of known genotypes. In other words, given a group of known genotypes for one trait, how many identical genotypes show the related phenotype? You might be surprised to learn that, for some traits, the phenotype might not occur as often as the genotype. For example, say everyone in population W carries the same allele combinations for a certain trait, yet only 85% of the population actually shows the phenotype expected from those allele combinations. The proportion of genotypes that actually show expected phenotypes is called penetrance. Thus, in the preceding example, the penetrance is 85%. This value is calculated from looking at populations whose genotypes we know.
In fact, large population studies are necessary for measuring penetrance, and studies of penetrance help us predict how likely it is that a trait will be evident in those who carry the underlying alleles. In general, when we know that the genotype is present but the phenotype is not observable, the trait shows incomplete penetrance. Basically, anything that shows less than 100% penetrance is an example of incomplete penetrance. Therefore, although the penetrance of a trait is a statistically calculated value based on the appearance of a phenotype among known genotypes, incomplete penetrance is simply a qualitative description about a group of known genotypes.
A specific example of incomplete penetrance is the human bone disease osteogenesis imperfecta (OI). The majority of people with this disease have a dominant mutation in one of the two genes that produce type 1 collagen, COL1A1 or COL1A2. Collagen is a tissue that strengthens bones and muscles and multiple body tissues. People with OI have weak bones, bluish color in the whites of their eyes, and a variety of afflictions that cause weakness in their joints and teeth. However, this disease doesn't affect everyone who has COLIA1 and COLIA2 mutations in the same way. In fact, some people can carry the mutation but have no symptoms. Thus, families can unknowingly transmit the mutation from one generation to the next through someone who carries the mutation but does not express the OI phenotype.
Incomplete penetrance examples such as OI demonstrate that even monogenic diseases do not have predictable expression patterns in a population. Is there a way to explain this unpredictability? Let's think about it. If two people have the same dominant mutation in COL1A1, why might only one of them actually display OI symptoms? Could it have to do with other genes that rescue the bad effect of a mutated collagen gene? Could it be that those who have OI simply express more mutated collagen than the person who is unaffected? To consider the possible explanations for incomplete penetrance, we have to remember how many steps there are between gene transcription and protein expression.
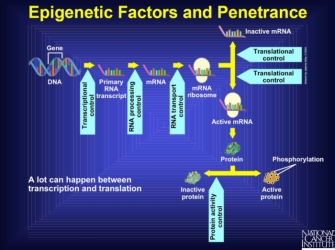
Note that the expression of other genes, such as transcriptional or translational regulators, can influence the final effect of a gene product. Anything that interferes with the pathway from transcription to protein activation is known as an epigenetic factor. Indeed, there are multiple points at which another gene product can intervene in the stages prior to the production of a protein. Interference at these stages might stop production entirely, create an altered form of the protein that might never be active, or do any number of other things that renders the gene silent. So, the final stage of an active protein reflects many different processes that lead to the amino acid sequence and ultimate protein shape, all of which can be interfered with by other genes. Furthermore, some genes can up- or downregulate rates of transcription, which changes the total amount of protein produced. Thus, genes that affect the final form and expression amount of another gene can be influential in the formation of the phenotype derived from the regulated gene (Figure 1).
So, if so many different possible modification points for a gene product exist, how can we narrow down the question of what causes incomplete penetrance? Interestingly, some scientists have actually tried to do this by observing how the genetic mutations that cause OI affect mice. These investigators inserted a mutated form of COL1A1 into mice and bred them so that they all contained this mutation. The mice were affected in similar ways to those with human OI: Many had severe bone weakness and multiple bone fractures, even at birth. In fact, when the researchers examined the mouse bones closely, they found that 70% of mice with the mutated COL1A1 gene showed evidence of OI (bone fractures); however, the remaining 30% appeared completely normal. In these mice with no OI phenotype, there was the same amount of COL1A1 expression as in those mice that did show the phenotype. Furthermore, the investigators used a purebred strain of mice that had little variability in their genomes to begin with. This means that the genetic context in which COL1A1 was expressed did not vary among the mice studied. Yet, despite the fact that all the mice had extremely similar genomes and all of them expressed the same amount of COL1A1, 30% of them did not show any OI phenotype. These results continue to be perplexing.
Therefore, even the powerful experimental techniques currently available cannot explain penetrance. The two most popular explanations for incomplete penetrance, genetic background and variable expression levels, did not explain the lack of phenotype in 30% of the mice (Pereira et al., 1994).
Source: http://www.nature.com/scitable/topicpage/phenotype-variability-penetrance-and-expressivity-573
0 Response to "Continuous Traits Are Often Influenced by Multiple Simple Mendelian Genes"
Post a Comment